What to do if you suspect that your work has been plagiarised? How to tackle authorship issues? Who can help you with these problems, and what kind of research integrity framework do we have in place in Finland?
Subject term search
The Nordic Committee on Bioethics and The Norwegian Biotechnology Advisory Board are hosting a webinar series on new uses of DNA in police work.
7.7.2020
On this page is a list of organisations that have committed themselves to following TENK's guidelines The Finnish Code of Conduct for Research Integrity and Procedures for Handling Alleged Violations of Research Integrity in Finland 2023 (PDF) also known
15.6.2020
The annual report 2019 of the Finnish National Board on Research Integrity TENK has been published.
24.4.2020
A Practical Model of the Self-Regulation of Academic Integrity: A Chinese-English Edition of the Code of Conduct for Research Integrity in Finland introduces the Finnish framework of research integrity for Chinese readers.
24.4.2020
Finnish National Board on Research Integrity TENK has produced a short video on the guidelines for ethical review in research with human participants.
22.4.2020
Violations of good research practices breach the principles of research integrity. They damage the quality and credibility of research and undermine research collaboration and authorship.
These actions may also be against the law, in which case they are
21.4.2020
In Europe, there are several different national approaches available for investigating violations of research integrity. There are also countries which still do not have any national framework. There are basically two courses of action in determining
21.4.2020
The Finnish National Board on Research Integrity TENK launched the research integrity adviser system in 2017. Research integrity advisers provide personal and confidential advice on research integrity to members of their organisation, and there are
21.4.2020
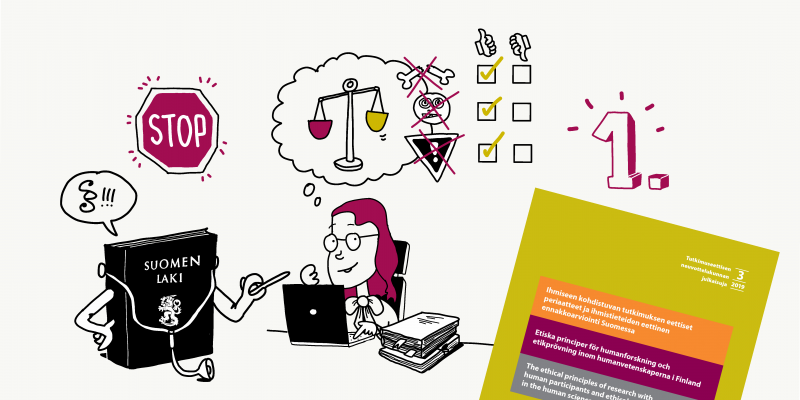
For research involving human participants, the Finnish National Board on Research Integrity TENK has issued a set of guidelines on the ethical principles to be followed.
21.4.2020
Ethical review in Finland depends on whether the research is medical or in the scope of human sciences.
Medical research
The Medical Research Act and Decree (488/1999) regulate medical research involving human beings.
According to the Act, medical
21.4.2020
Alleged violations of research integrity (so-called RI violations) are investigated in the research organisation where the alleged violation has taken place. The process of handling alleged violations of research integrity, or the RI process, must be